Chemical modifications only shift the siRNA seed profile

In the last post, we saw that chemically modified ON-TARGETplus siRNAs still have a strong seed effect.
The seed-based off-target effects (measured by correlation of reagents with the same 7mer seed) were as strong for chemically modified ON-TARGETplus (R = 0.50) and Silencer Select (R = 0.59) as what we typically see with unmodified siRNAs (Qiagen, siGENOME, or Silencer).
Chemical modification must not prevent seed-based target recognition, because RISC uses the seed to scan the transcriptome for target sites. Because of how RISC presents the guide strand seed region for target scanning, the binding energy for finding an on-target site (19-base complementarity) versus an off-target site (6/7-base complementarity) is nearly the same. It’s not like a microarray oligo, where more extensive complementarity leads to stronger binding. The seed is driving this site recognition, so any modification that eliminates its binding will make the siRNA ineffective.
The chemical modifications added by Ambion and Dharmacon do not prevent seed binding, but instead change the efficiency of different bases at certain positions, in effect changing the seed profile of off-target sites.
The following heatmap shows the cell viability scores from 9 genome-wide siRNA screens. The average viability score for all siRNAs with a specific base at a specific position was calculated (shown are guide positions 1-9). If the value is red, it means siRNAs with the base at that position tend to be more lethal.
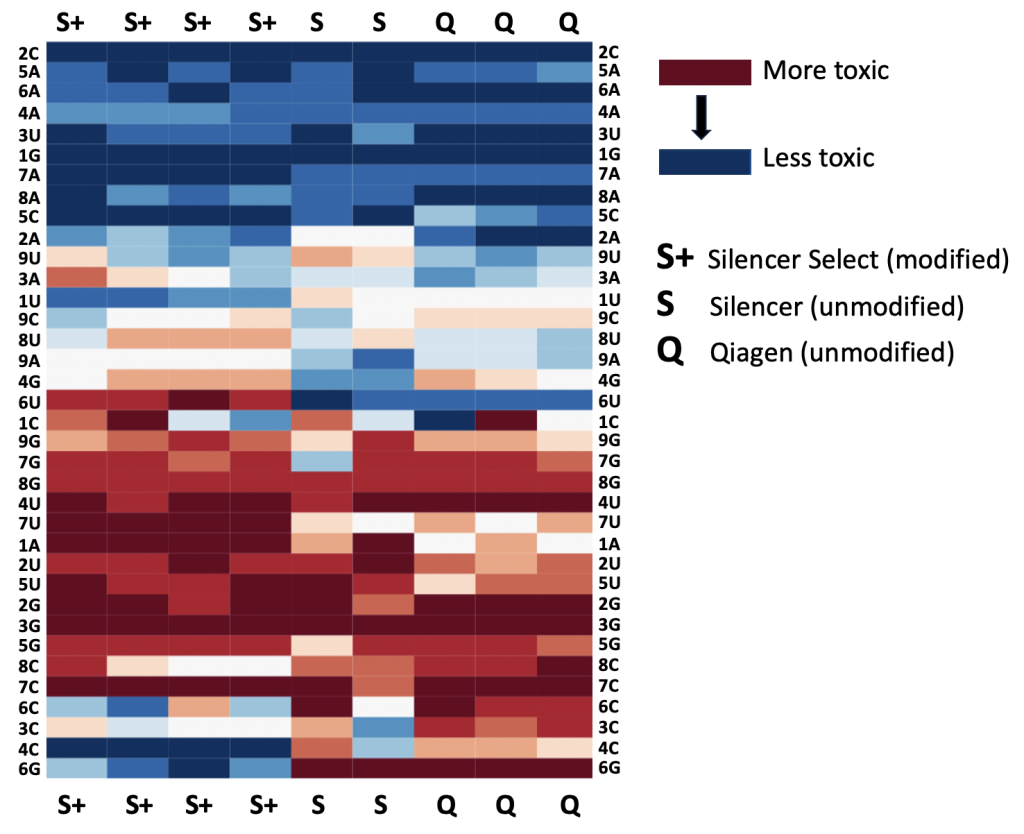
The first 4 columns are from screens using chemically modified, Silencer Select siRNAs (S+). The next 2 columns are from screens using unmodified, Silencer siRNAs (S). And the last 3 are from screens using unmodified, Qiagen siRNAs (Q).
We see that for some bases (e.g. 2C, top row), siRNAs tend to be non-toxic regardless of whether or not they are chemically modified (S+, S, and Q all show deep blue).
But there are other positions where the chemically modified siRNAs are very different from the unmodified siRNAs.
For example, the bottom row shows that 6G tends to be very toxic in unmodified siRNAs, but is not toxic in Silencer Select (chemically modified) siRNAs. On the other hand, 6U (towards the middle row) looks to be toxic for Silencer Select siRNAs but have the opposite effect for unmodified siRNAs.
Whatever the chemical modification for Silencer Select is (has not been made public), it appears to make seed off-targets stronger when position 6 is a U, and weaker when position 6 is a G.
If we compare the effect on cell viability of Silencer Select vs ON-TARGETplus siRNAs from the Tan and Martin screen (subject of last post), we also see strong differences in the effect of having a U or a G at position 6.
The following plot shows the toxicity rank of seed bases in Silencer Select siRNAs vs ON-TARGETplus siRNAs. Bases towards the origin (e.g., 2C) tend to make siRNAs non-toxic for both types, whereas bases towards the top right (e.g., 2G) tend make make siRNAs toxic for both types. Bases that fall off the diagonal tend to be toxic for one type and non-toxic for the other.
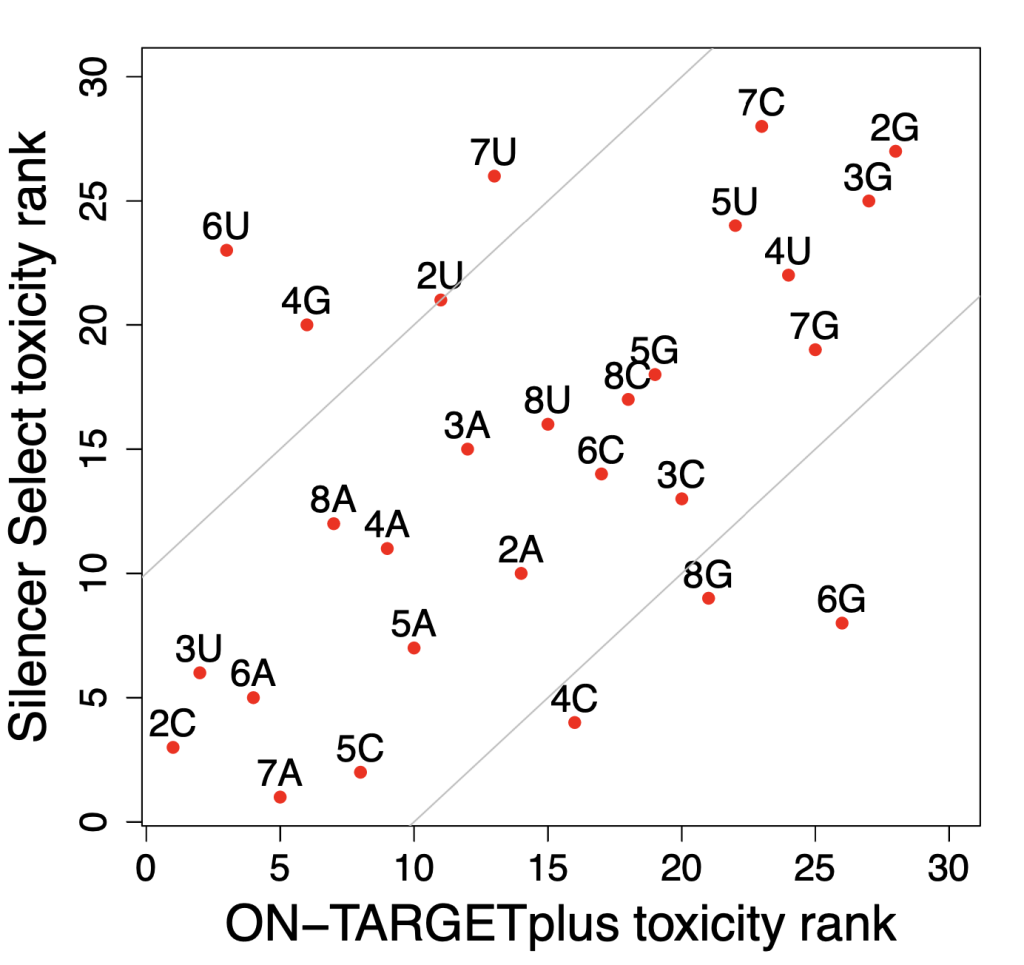
We see that 6U is toxic for Silencer Select siRNAs (as also seen in the heat map) and ON-TARGETplus siRNAs, like the unmodified siRNAs from the heat map, tend to be non-toxic. And the effect is similar to the heat map for 6G: toxic for Silencer Select and non-toxic for ON-TARGETplus (and unmodified in heat map).
Conclusion
Chemical modification does not get rid of seed effects, as evidenced by the strong phenotypic correlation of modified siRNAs with the same seed sequence. Rather, modifications tend to change the effectiveness for specific bases in eliciting seed-based silencing.
One suggestion would be to design a chemically modified siRNA library that avoids bases that tend to be toxic (e.g., 6U for Silencer Select).
However, there are a few problems:
- The heat maps and scatterplot only show tendencies. There is still variation within those positions. While 2G tends to be non-toxic for Silencer Select, there are still lots of toxic siRNAs with that sequence.
- Bases that reduce toxicity may be doing so because they tend to reduce target recognition. For example, 2C is also associated with poorer on-target silencing. Using only 2C for siRNAs could thus result in a library that is not as efficient at on-target silencing.
- Finally, these suppliers have already produced their siRNA libraries. i.e., those bases have already been used.
The only reliable way to both reduce the off-target effect (via dilution of seeds) and maintain robust on-target silencing is by using siRNA pools (siPOOLs).